The aim of green chemistry is to reduce chemical related impact on human health and virtually eliminate contamination of the environment through dedicated, sustainable prevention programs. Green chemistry searches for alternative, environmentally friendly reaction media and at the same time strives to increase reaction rates and lower reaction temperatures.
The green chemistry concept applies innovative scientific solutions to solve environmental issues posed in the laboratory. Paul T. Anastas, an organic chemist working in the Office of Pollution Prevention and Toxins at the EPA, and John C. Warner developed the Twelve Principles of Green Chemistry in 1991. These principles can be grouped into Reducing Risk and Minimizing the Environmental Footprint.
Reducing Risk in the Laboratory
Sigma-Aldrich is dedicated to providing alternative products designed with the health and safety of its employees, customers, and the public in mind.
- Use Safer Chemicals – Utilize performance chemicals that have the lowest levels of toxicity.
- Design Less Hazardous Synthesis Methods – Where feasible, make use of synthetic or biosynthetic methods that pose little or no toxicity to human health and the environment.
- Use Safer Solvents and Reaction Conditions – Search for the most up-to-date information on green solvents that will optimize your process and provide a safer working environment.
- Accident Prevention – Select substances that minimize the potential for explosions, fires and chemical releases into the environment.
Minimizing the Environmental Footprint
- Waste Minimization and Prevention – Develop chemical synthesis techniques, which reduce or prevent waste. It is better to prevent waste than to clean it up after its creation.
- Use of Catalysts Instead of Stoichiometric Quantities – Catalytic reactions inherently use smaller quantities of chemicals to carry out a specified transformation.
- Reduce the Use of Chemical Derivatives – The use of protecting groups or other forms of temporary modification of a functionality adds to the total waste incurred in a synthetic route.
- Synthetic Efficiency (Atom Economy) – An efficient chemical process ensures the maximum amount of your starting materials is used in the final product so that no atom is wasted.
- Taking Advantage of Chemicals Designed for Degradation – Reduce the effect on the environment by using chemicals that are designed to be biodegradable.
- Establishment of In Process Controls for Pollution Prevention – To avoid the formation of hazardous substances, adopt real-time analysis and in process monitoring during synthesis.
- Use of Renewable Feedstock – Use raw materials or renewable feedstock (waste from other processes or products derived from agricultural streams) whenever technically or economically feasible.
- Encourage Energy Efficiency – The realization of the economical and environmental impact of energy use in a chemical process and the development of alternative means to reduce the impact.
If you are looking to protect your health and environment in your lab, you could benefit from taking a look at Sigma-Aldrich product range, which includes:
- alternative energy
- biodegradable polymers
- boron compounds and modifications
- catalysers
- flow chemistry
- a range of solvents and reagents
- ionic liquids
- novozymes
- returnable container programme for solvents.
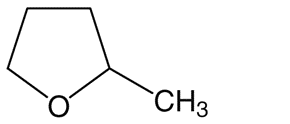
2-Methyltetrahydrofuran (2-MeTHF)
A Truly Green Alternative to Dichloromethane (DCM) and Tetrahydrofuran (THF)
2-MeTHF is derived from renewable resources such as corncobs and bagasse. When used as an organometallic solvent, 2-MeTHF offers both economical and environmentally friendly advantages over Tetrahydrofuran.
Features & Benefits:
- Aprotic polar solvent
- Resembles Toluene in physical properties.
- Grignard reagents tend to be more soluble in 2-MeTHF than in THF.
- Forms an azeotrope rich with water, therefore can be more easily dried than THF or DCM.
- Limited miscibility in water (4.1g/100g at 23°C) allows easy separation and recovery from water reduces the waste stream.
- Higher boiling point (80°C) compared to THF. Higher reaction temperature reduces overall reaction time.
- Low heat vaporization means less solvent loss during reaction reflux. Besides, this saves energy during distillation and recovery.
Alternative to Tetrahydrofuran for Organometallic Reactions, such as:
- Grignard
- Reformatskii (Reformatsky)
- Lithiation
- Hydride Reduction
- Metal-Catalyzed Coupling (Heck, Stille, Suzuki)
Alternative to Dichloromethane for Biphasic Reactions, such as:
- Alkylation
- Amidation
- Nucleophilic Substitution Reaction
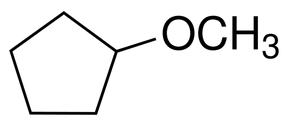
Cyclopentyl methyl ether (CPME)
Environmentally Friendly Alternative to Tetrahydrofuran, tert-Butyl methyl ether (MTBE), 1,4 Dioxane and other ether solvents.
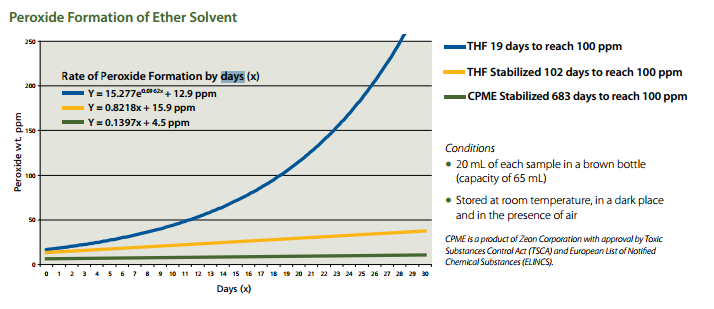
CPME provides a green solution for those looking to improve their chemical process by not only minimizing the solvent waste stream, but also improves laboratory safety due to CPME’s unique composition which resists the formation of peroxides.
Features & Benefits
- More stable than THF and 2-MeTHF (stabilizer required):
- Resists peroxide formation,
- Reduces the frequency of peroxide testing.
- Novel hydrophobic ether solvent:
- Useful in many organometallic reactions,
- Provides better yields and higher selectivity over THF.
- Forms an azeotrope rich with water, therefore can be more easily dried than THF and 2-MeTHF.
- Limited miscibility in water (1.1g/100g at 23°C) allows easy separation and recovery from water reduces the waste stream.
- Higher boiling point (106°C) compared to THF and 2-MeTHF means higher reaction temperature, which reduces overall reaction time.
- Low heat vaporization means less solvent loss during reaction reflux. Besides, this saves energy during distillation and recovery.
CPME Applications:
- Asymmetric Michael Alkylation
- Michael addition of R2CuLi
- Alkylation of chiral amide
- Glycosidation
- Asymmetric hydrogenation of NaBH4
- Hydrosilylation by Ru cat
- Nucleophilic Reactions
- Amide synthesis by the reaction of acid chloride with amine
- Sillylation and desillylation
- Reaction of Carbon anion with aldehyde
- Debenzylation
- Alkylation of amine
- Selective methylation of phenols
- Bromination of alcohol with PBr3
- Sulfonylchloride synthesis by the reaction of sulfonic acid with PCl5
- Reactions using Metals
- Reaction of ketone using NaBH4
- Reaction of acetylenes with Ti(OR)4
- Reaction using n-BuLi or Lithium Diisopropyl Amide
- Radical cyclization of trichloroacetate using Cu cat
- Reduction of ethyl benzoate using Lithium Aluminium Hydride
- Formation of sodium dispersion
- Intramolecular ene reaction using ZnCl2.